Determination of chlorine by DPD methods
for more information
subscribe here
for more information
subscribe here
Introduction :
We use DPD as indicator because of it's high
sensitivity for chorine ,DPD reacts with
chlorine producing a bright red color ,so we can use it by two methods colorimetric or titration with
ferrous ammonium sulphate (FAS) Fe(NH4)2SO4
.
This DPD
methods very accurate but the
main defect that it is
can not directly determine the
concentrations exceeds 5 mg/l . this can achieved by using dilution
DPD oxalate
A)colorimetric methods.
since the degree or the strength of red color that is yields from reaction between DPD and
chlorine proportional to the concentration of chlorine we can
compare the color which developed when DPD added to a sample
and standard colors known it's
concentration
The comparison may takes place either by simple device
such as above in which a cycle has a graduated red or pink color ,each degree
of color equivalent to Known concentration
or by spectrophotometric
methods
The
simple comparison colorimetric method:
Preparations:
1) prepare DPD solution as the following :
Dissolve 1 g DPD oxalate in little amount of
distillated water containing 8 ml 1:3 sulfuric acid and 200 mg EDTA complete to 1 L freshly boiled and cooled
distillated water (must be sure that is free from chlorine ) , store in brown
or dark bottle
2)prepare phosphate buffer :
a)Dissolve 24 g Na2HPO4 and 46 g
KH2PO4 in little amount distillated water
b)dissolve 800
mg EDTA in 100 ml distillated water
mix the phosphate solution and EDTA solution ,dissolve
well ,and complete to 1 L
see altrnative methods for preparation this buffer solution
Procedure:
Add 5 drops Buffer solution +5 drops DPD solution to sampling tube , then add to it 10 ml
sample water and put it in comparison device , read the concentration of the
color strength equals to the strength of color developed in sampling tube .
The spectrophotometric comparison colorimetric
method
Prepare DPD and buffer solutions as above:
1)Prepare known concentrations of chlorine (use
thiosulphate or DPD tablets or DPD – FAS method as we see)
2)set the spectrophotometer on 515 nm and blank it with distillated water
3) add 5 drops buffer +5 drops DPD +10 ml known
concentration chlorine solution to
spectrophotometer cell and read the corresponding absorbance
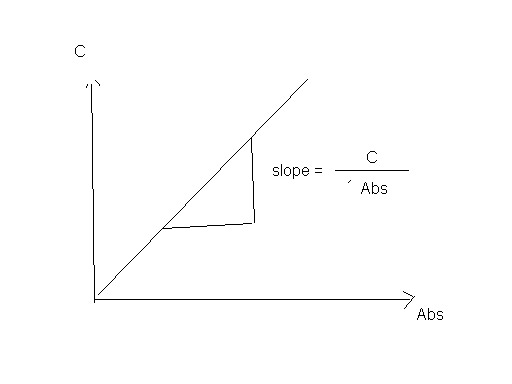
Plot a curve between absorbance in horizontal axes and
concentration in vertical axes and detect the slope as the following
Concentration = slope *Abs
|
B) Titration Method (Ferrous ammonium sulphate
method)
Preparations:
1)Prepare
DPD and buffer solutions as above
2)Prepare standard ferrous ammonium sulphate solution (FAS)
Fe(NH4)2SO4 as the following :
Dissolve 1.106 g
Fe(NH4)2SO4.6H2O in
distillated water containing 1 ml 1:3 sulfuric acid ,then complete to 1 liter freshly boiled and
cooled distillated water .
3)KI crystals
or 0.5% KI solution (500 mg KI in 100ml distillated water )store in
brown bottle
Procedure:
a) Free chlorine and chloramines
add 5 ml buffer + 5 ml DPD solution in conical flask ,
mix , add 100 ml sample and mix well.
i) Free chlorine
titrate rabidly with standard FAS solution until red
color discharged (reading A).
ii) Monochloramine ( NH2Cl).
add very small crystal of KI (about 0.5 mg) or 0.1 ml
(about 2 drops) KI solution ,complete titration
with FAS solution until red color
discharged again.(reading B).
iii)Dichloramine. ( NHCl2).
Add several crystals KI (about 1 g) mix, stand
for 2 minutes ,continue titration until
red color discharged too (reading C).
iv) Trichloramine. (NCl3)
add very small crystal of KI (about 0.5 mg) or 0.1 ml
(about 2 drops) KI solution +100 ml sample in conical flask ,add the content to
another conical flask containing 5 ml
buffer + 5 ml DPD solution ,titrate rabidly with FAS (reading N)
.
b) Total chlorine (free +combined) in one
reading
add the full amount of KI at the start of titration
+100 ml sample in flask ,add the content to another conical flask
containing 5 ml buffer + 5 ml DPD
solution ,titrate rabidly with FAS
Calculations:
Determine the free chlorine, mono, di and tri
chloramines as explained in the following table:
NCl3 present
|
NCl3 absent
|
Reading
|
Free Cl
NH2Cl
NHCl2 +1/2 NCl3
Free Cl
+1/2 NCl3
NCl3
NHCl2
|
Free Cl
NH2Cl
NHCl2
|
A
B-A
C-A
N
2(N-A)
C - N
|



هناك تعليق واحد:
I loved as much as you will receive carried out
right here. The sketch is attractive, your authored material stylish.
nonetheless, you command get bought an edginess over that you wish
be delivering the following. unwell unquestionably come more formerly again as exactly the same nearly a lot often inside case you
shield this increase.
Here is my blog post nude man
إرسال تعليق